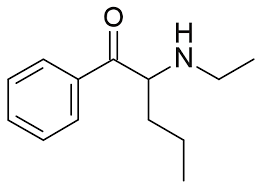
NEP (N-Ethylpentedrone) is a synthetic stimulant belonging to the cathinone class of compounds, which are chemically related to amphetamines. It is a derivative of pentedrone, with an ethyl group attached to the nitrogen atom, which influences its pharmacological properties. NEP is known for its potential stimulating and euphoric effects, making it a subject of research in neuropharmacology.
Key Characteristics of NEP:
- Chemical Structure: NEP has a beta-keto group on its backbone, a hallmark of cathinones, and an ethyl substitution on the nitrogen atom, which differentiates it from its parent compound, pentedrone.
- Mechanism of Action: It likely acts as a releasing agent or reuptake inhibitor of monoamines, primarily dopamine and norepinephrine, leading to stimulant-like effects.
- Effects: Research indicates that NEP may produce euphoria, increased energy, enhanced focus, and elevated mood similar to other cathinones.
Research Applications:
NEP is primarily studied in laboratory settings to:
- Understand Neurotransmitter Interactions: Investigate how it affects dopamine, norepinephrine, and serotonin systems.
- Explore Structure-Activity Relationships (SAR): Analyze how the N-ethyl substitution impacts potency and receptor binding compared to pentedrone and other cathinones.
- Compare with Analogues: Examine its effects relative to related compounds like α-PVP, pentedrone, and ethylone.
Safety and Regulation:
- Potential Risks: Adverse effects may include overstimulation, anxiety, insomnia, cardiovascular strain, and risk of dependency.
- Legal Status: NEP is categorized as a research chemical in many regions, restricting its use to scientific and laboratory purposes. It is not approved for human or veterinary use.
Important Information:
NEP is not for human consumption and is intended solely for laboratory research. It should only be handled by qualified researchers in controlled environments.

Reviews
There are no reviews yet.